EpiFlaMe – Inflammatory Memory in Epithelia: organ imprint and cancer
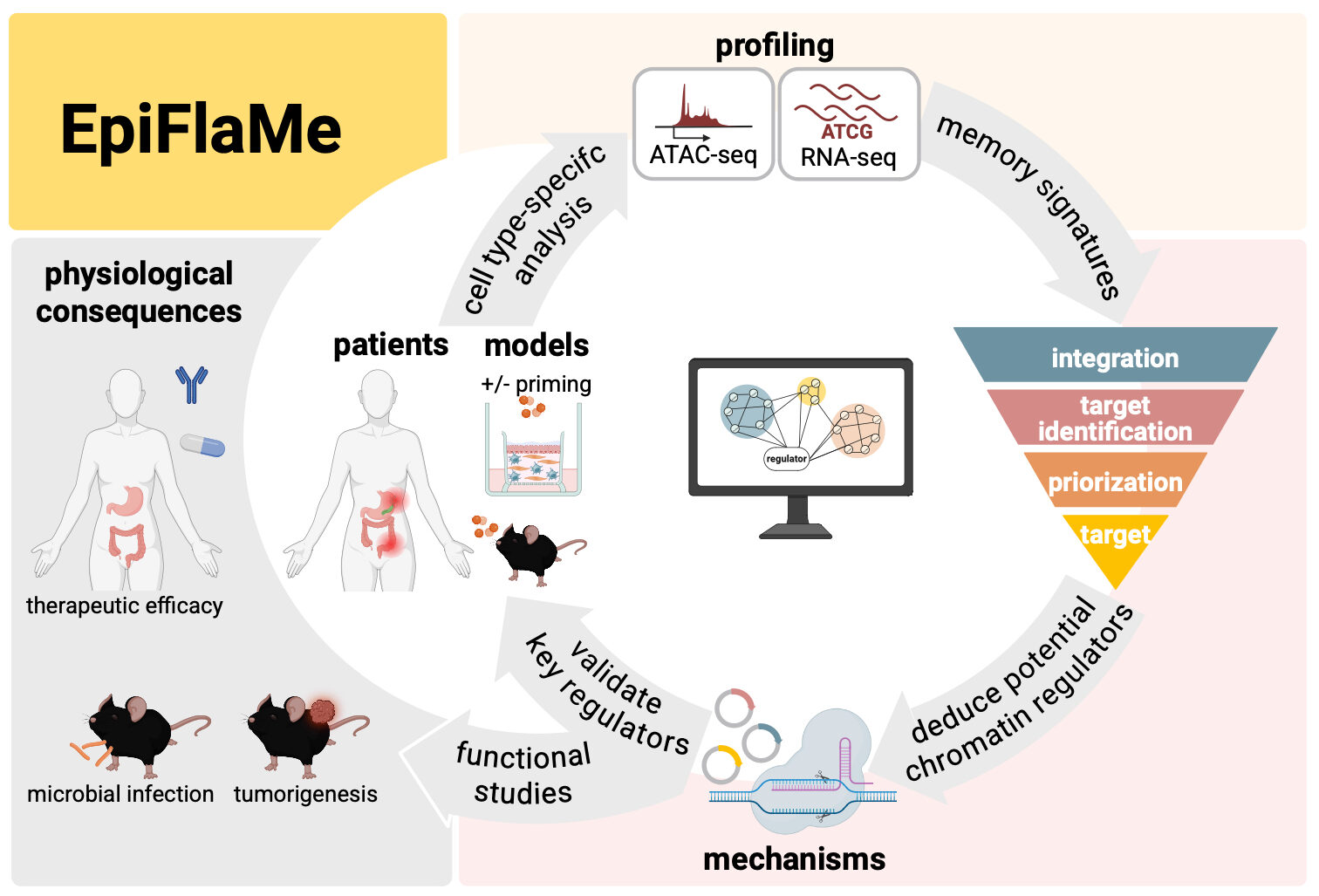
Wider research context: Cells remember past insults on an epigenetic level – an ability termed ‘trained immunity’ in innate immune cells and ‘inflammatory memory’ in epithelial cells and other structural cells. This strongly influences secondary immune responses and cancer development. Importantly, while the epigenetic landscape of cells in steady state is distinctly shaped by the organ environment, the impact of the organ environment on epigenetic changes (memory) after inflammation is poorly understood, including the epigenetic mechanisms and regulators that establish inflammatory memory. A detailed and systematic understanding of the organ-specific molecular and functional consequences of memory formation is required for effective therapeutical interventions.
Hypothesis: We hypothesize that inflammatory memory of structural cells is highly organ-specific with significant implications for subsequent inflammatory responses and cancer development. Thus, we will (i) reveal cell type- and organ-specific molecular signatures of inflammatory memory and trained immunity (ii) (ii) identify the key epigenetic regulators and mechanisms of memory formation and (iii) elucidate the consequences of inflammatory memory for infection and cancer.
Approach: We will expose composite organoid cultures and mice to cytokines or bacterial infections to induce inflammatory memory and systematically compare the resulting transcriptional and epigenetic changes across (i) cell types (epithelial cells, fibroblasts, and myeloid cells) and (ii) different organs (skin, lung, stomach, colon, and liver). We will identify regulators of organ-specific memory using CRISPR screens, elucidate the functional consequences of memory on pathogen clearance, and assess potential interactions of inflammatory memory and oncogenic pathways that may shift the balance from healthy tissue towards cancer.
Innovation: Inflammatory processes in epithelial cells critically contribute to chronic inflammation and the formation of carcinomas, which originate from epithelial cells and account for approximately 80% of cancer deaths worldwide. Our consortium will generate the first systematic molecular map of inflammatory memory of epithelial cells, fibroblasts and myeloid cells across organs and gain mechanistic insights into the regulation of memory. This will provide a basis for future therapeutics to treat chronic inflammatory diseases and to interfere with tumor formation in an organ-specific manner.
Added value: The consortium combines experts in immunology, microbiology, epithelial inflammation, cancer (all with established in vivo mouse models and organoid co-culture systems), high-throughput profiling, and computational systems biology. This will enable us to systematically study organ-specific memory formation and its consequences for inflammatory processes and cancer development.
Primary researchers involved: The research groups of Fritz Aberger, Nikolaus Fortelny, Iris Gratz, Thomas Krausgruber, Dirk Schmidt-Arras, Philipp Starkl, and Silja Wessler will each hire one PhD student, which will be supported by two consortium-wide bioinformaticians. Additionally, two research technicians (funded by the State of Salzburg) and in-kind technical and scientific staff from the respective institutions will work on EpiFlaMe.





