Collagen degradation in the spotlight: How bacterial collagenases cleave this hard-to-digest protein
Collagen degradation in the spotlight: How bacterial collagenases cleave this hard-to-digest protein
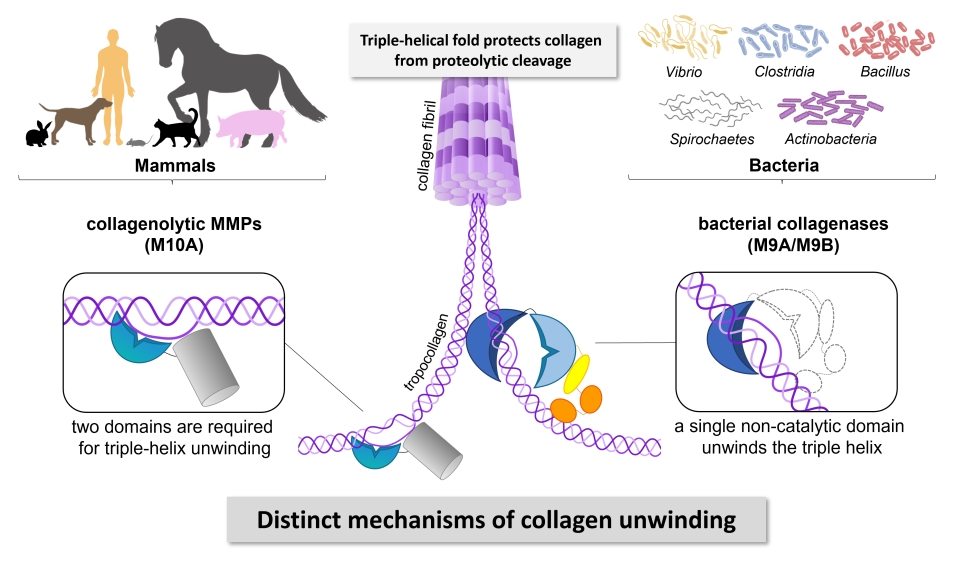
Bacterial collagenases play an important role in the virulence of certain harmful bacteria from Clostridium, Bacillus, Spirochaetes, and Vibrio. They also find widespread application in industry, research and clinics. Despite their importance, the exact way how these enzymes break down collagen has remained a mystery. Scientists headed by Dr. Esther Schönauer at the Department of Biosciences and Medical Biology (PLUS) investigated collagenase G (ColG) from Clostridium histolyticum and revealed that ColG interacts with and processes different physiological forms of collagen in distinct ways. Whether it is fibrillar or soluble collagen, the enzyme adapts its behaviour accordingly. Moreover, by employing biochemical techniques and circular dichroism studies, they have pinpointed a surprising discovery: the presumed non-catalytic activator domain of ColG in fact acts as triple helicase, unwinding collagen molecules locally and temporarily.
This research holds significant implications. Collagen is a critical component of the extracellular matrix in humans and other mammals, providing strength and structure to all tissues. Bacterial collagenases, belonging to the metalloprotease family M9, possess the unique ability to break down collagen at multiple sites despite its tough triple-helical structure. The Austrian Science Fund (FWF)-funded research project shed new light on how these enzymes achieve this feat, uncovering a mechanism distinct from that of human collagen-degrading enzymes. Understanding the intricacies of bacterial collagenases opens up new possibilities for developing targeted inhibitors to combat bacterial infections. By disrupting the specific mechanisms these enzymes use to attack collagen, this may pave the way for more effective treatments against pathogenic bacteria.
The results were published in the prestigious scientific journal Proceedings of the National Academy of Sciences (PNAS):
Title: A conserved strategy to attack collagen: The activator domain in bacterial collagenases unwinds triple-helical collagen.
Abstract incl. Significance statement (Extract from PNAS):
Bacterial collagenases are important virulence factors, secreted by several pathogenic Clostridium, Bacillus, Spirochaetes and Vibrio species. Yet, the mechanism by which these enzymes cleave collagen is not well understood. Based on biochemical and mutational studies we reveal that collagenase G (ColG) from Hathewaya histolytica recognizes and processes collagen substrates differently depending on their nature (fibrillar vs. soluble collagen); distinct dynamic interactions between the activator and peptidase domain are required based on the substrate type. Using biochemical and circular dichroism studies, we identify the presumed non-catalytic activator domain as the single-domain triple helicase that unwinds collagen locally, transiently and reversibly.
Significance Statement
Collagens form the resilient backbone of the mammalian extracellular matrix. Only few proteases are able to digest collagen because of its tight triple-helical fold and high content of prolines and hydroxyprolines. Bacterial collagenases of the metalloprotease family M9 can efficiently degrade triple-helical collagen into small peptides. Yet, their mechanism of action is not well understood. We demonstrate that the activator domain of bacterial collagenases single-handedly unwinds collagen triple helices, enabling their subsequent cleavage by the peptidase domain. Our findings reveal that bacterial collagenases employ different mechanisms to recognize and unwind collagen than human collagenolytic MMPs. This discovery opens a new avenue for the development of highly selective inhibitors targeting bacterial collagenases.





