Research at the Energy Materials Group
Many of today’s obstacles and opportunities for next-generation electrochemical energy storage lie in the complexity of the multiphase materials systems. The physico-chemical interplay of individual materials components defines the overall system properties – similar to the field of systems biology. Electrochemical systems with identical chemical but different structural compositions can show entirely new properties. An intriguing example is the reversible electrodeposition of solid iodine nanoclusters in the confinement of carbon nanopores with sizes < 1 nm ( Nature Communications 2020). The same system with slightly larger pores would not allow for persistent deposition of solid iodine and, thus, significant storage of energy. Hence, the properties and function of next-generation energy storage are not only rooted in the chemistry of its individual chemical compounds. Instead, they are defined by reaction mechanisms, structure, and transport in conjunction. Rational design criteria, therefore, rely on a quantitative and systemic understanding across length scales.
Our strategy to deal with this complexity is two-fold:
1. The bottom-up approach

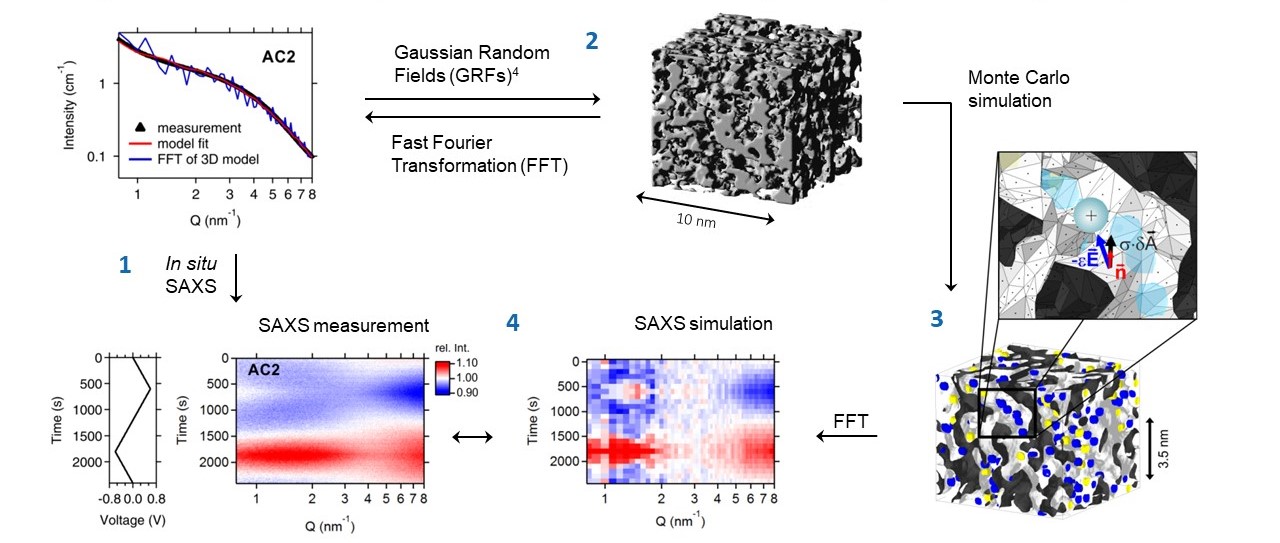
We aim to establish new experimental methods and methods for data analysis. We will use cryo-electron microscopy, operando small angle scattering, and machine-learning-supported stochastic modeling to visualize and quantify the structural evolution during electrochemical operation. We combine the advantages of time-resolved integral structural information, local element-specific microscopy, and quantitative data analysis to recognize even subtle structural transitions with a seamless sensitivity from the atomic to the µm scale.
METROLOGIES
We aim to understand the fundamentals of solid-state and solid-liquid-solid electrochemical conversion of sulfur and more generally poorly conducting high-capacity materials. Equivalent to microelectronics, batteries will be understood as mesoscopic heterostructures, with all the possibilities to alter transport, storage, and charge transfer by mesoscopic structuring. Our research may overturn the common belief that nominally insulating materials cannot be used in high-performance batteries. This could significantly impact how we select future high-energy storage materials. By understanding the interaction of individual compounds, we will design mesoscopic materials structures to control transport, charge transfer, and phase transformations – and ultimately achieve new system properties.
FUNDAMENTALS
Our research may have an important impact on society by realizing high-energy batteries with low cost and abundant sulfur and without critical raw materials. The Li-S battery cathodes, as proposed in the ERC Starting Grant project SOLIDCON, may store ~ 4 times more charge per total electrode mass and ~ 2.5 times more charge per total electrode volume than today’s Li-ion battery cathodes. This translates to ~ 2.2 times higher gravimetric and ~ 35 % higher volumetric energy densities. With more and more advanced manufacturing, the Li-ion battery price is increasingly defined by the raw materials costs of the expensive transition metals. Me-S batteries would eliminate the dependency on transition metals with volatile prizes (Ni, Co) and problematic extraction (Co). Hence, with an increased energy density and the low raw materials cost of S, we could significantly reduce the costs per stored kWh in future batteries.
DEVICE ENGINEERING
Our research may have an important impact on society by realizing high-energy batteries with low cost and abundant sulfur and without critical raw materials. The Li-S battery cathodes, as proposed in the ERC Starting Grant project SOLIDCON, may store ~ 4 times more charge per total electrode mass and ~ 2.5 times more charge per total electrode volume than today’s Li-ion battery cathodes. This translates to ~ 2.2 times higher gravimetric and ~ 35 % higher volumetric energy densities. With more and more advanced manufacturing, the Li-ion battery price is increasingly defined by the raw materials costs of the expensive transition metals. Me-S batteries would eliminate the dependency on transition metals with volatile prizes (Ni, Co) and problematic extraction (Co). Hence, with an increased energy density and the low raw materials cost of S, we could significantly reduce the costs per stored kWh in future batteries.
2. The data-driven approach
We aim to integrate machine learning into the experimental workflow of battery testing. Enhancing the performance of battery systems by simple experimental parameter variation is often impossible due to the large multidimensional parameter space. Using closed-loop Bayesian optimization, for example, battery systems can be optimized significantly faster – without knowing all details of the underlying physics or testing thousands of battery cells.
Closed-loop optimization of battery performance with machine-learning

Parts of the above Figure adopted from F. Häse et al., Phoenics: A Bayesian Optimizer for Chemistry, ACS Cent. Sci. 2018, 4, 9, 1134–1145. This is an unofficial adaptation of an article that appeared in an ACS publication. ACS has not endorsed the content of this adaptation or the context of its use.