40Ar / 39Ar geochronology WHAT IS IT?
Geochronology is that research in geology, with the absolute age determination (as opposed to “relative” age determination which uses the fossil record) bschäftigt from gelogischen processes by means of physical methods.40Ar / 39Ar is in this context, for either of these methods, which is also available in the AG Geology University of Salzburg available. The method is based on the decay of naturally occurring radioactive isotopes whose ratio may be intended for a different stable isotope using a mass spectrometer. The Salzburger laboratory we call ARGONAUT; ARGON is used for the isotopic system, AUT for Austria.
1) In which minerals Argon occurs?
Argon (. Chem element Ar) is a noble gas, which occurs not only in the atmosphere, but also in various minerals; namely in those minerals, in which also the element potassium (chem. Element K) is involved in the formation of the crystal structure, so for example, in K-feldspar, muscovite, biotite, hornblende, ….
2) Why is there a relationship between K and Ar?
In nature there are different K-isotopes. Isotopes are variations of a chemical element with the same number of protons (p), but different numbers of neutrons (n) in the atomic nucleus. One of the three naturally occurring isotopes of K, the isotope with mass number 40, 4019K, (alos that element, which consists of 19 p and 21 n) is radioactive. That is, 4019K is unstable and decomposes into an isotope of another element, namely to the Ar isotope 4018Ar (consisting of 18 p and 22 n is composed). So: Argon (namely 4018Ar) is formed by the natural decay of the naturally occurring radioactive potassium isotope 4019K in Mineral.
3) And where does the 39Ar?
The other Ar isotope, which is necessary for the age determination using the 40Ar / 39Ar method, which 3918Ar is produced by irradiating the mineral sample in a reactor from the stable isotope K-3919K. You need it in order to have any idea how much K is ever in mineral inside.
4) How can you distinguish and measure 39Ar 40Ar from?
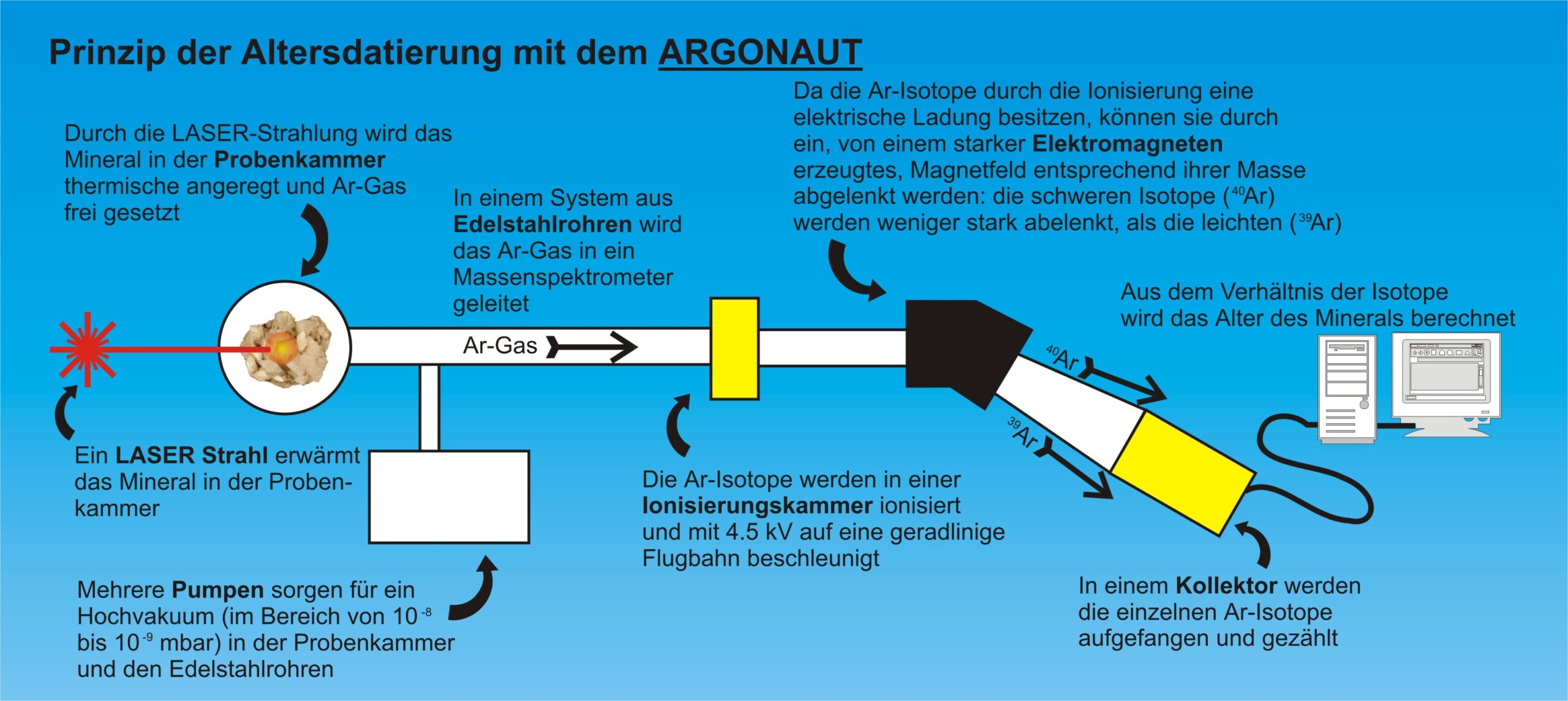
Protons and neutrons have mass, they “weigh” something. And isotopes with different numbers of protons and neutrons (ie different mass numbers m = p + n), are therefore different weights – the more protons and / or neutrons, the more difficult the isotope! And different heavy isotopes, with the aid of a mass spectrometer from each separation and count afterwards – that is the principle of mass spectrometry!
5) And how do you get the argon out of the mineral?
For this, the mineral is in the high-vacuum system that is connected to the mass spectrometer, is introduced, and thermally heated with a laser beam to melt them.





