
Bacterial HtrA proteases in the disruption of the epithelial barrier
The epithelium is the first barrier met by pathogens. Hence, successful pathogens developed fascinating strategies to interfere with the protective barrier. Depolarization and migration of epithelial cells imply the disruption of cell adhesion junctions (AJs) comprising a protein complex of E-cadherin, beta-catenin, p120, alpha-catenin, etc.. We analyze the disintegration of E-cadherin-mediated AJs and found that loss of E-cadherin-dependent cell-cell contacts is entirely independent of H. pylori CagA. High-temperature requirement A (HtrA) was identified as a new secreted virulence factor of H. pylori, which cleaves-off the ectodomain of the cell-adhesion protein E-cadherin. E-cadherin shedding disrupts epithelial barrier functions allowing H. pylori to access the intercellular space.
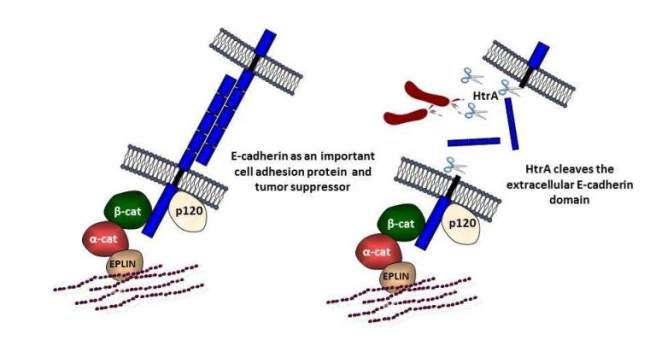
HtrA/DegP proteases are not only secreted by H. pylori. A wide range of bacteria also expresses the HtrA/DegP homologs DegQ and/or DegS, which significantly differ in structure and function. E-cadherin shedding is observed upon infection with the bacterial pathogens Campylobacter jejuni, enteropathogenic Escherichia coli (EPEC), Salmonella enterica subsp. Enterica (S. Typhimurium), Yersinia enterocolitica, or Proteus mirabilis, which express different combinations of HtrAs. Importantly, comparing the caseinolytic and E-cadherin cleavage activities of HtrA/DegP, DegQ and DegS proteins revealed that DegP and DegQ homologs from Gram-negative pathogens, but not activated DegS, cleaved E-cadherin as a substrate in vitro. This indicates that E-cadherin cleavage is confined to HtrA/DegP and DegQ proteins representing an important prevalent step in bacterial pathogenesis.
Important publications:
- Schmidt et al., 2016, Sci Rep
- Abfalter et al., 2016, Cell Commun Signal
- Hoy et al., 2010, EMBO Rep





