
Working Group Calorimetry/petrology
Research focus:
Synthesis, thermodynamic properties, and phase diagram determination of various materials, especially geologically important minerals and solid solutions, industry-relevant materials (cement phases and Li-ion conductors), metals and alloys, and glasses.
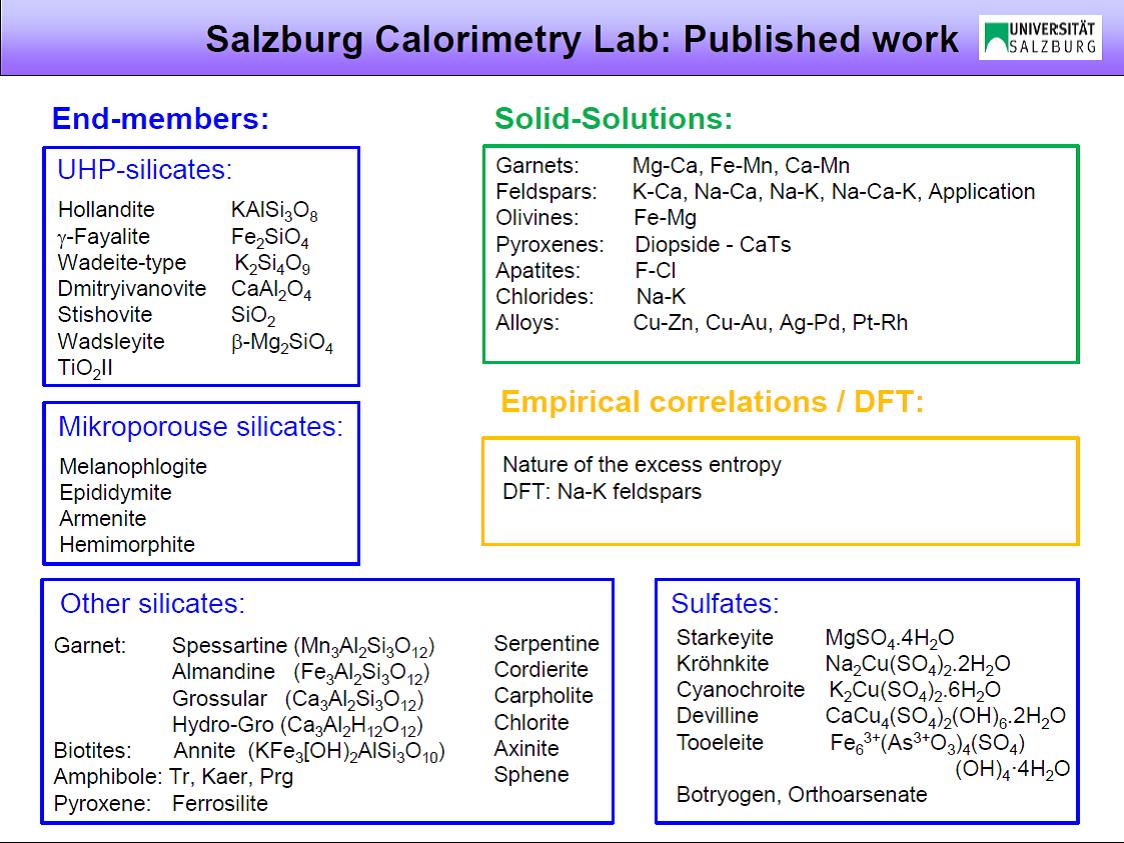
Heat capacity, a key thermodynamic property, is experimentally determined via calorimetry from low to high temperatures. This work started in 2003 with the establishment of the Salzburg Calorimetry Lab. From these measurements other thermodynamic properties, namely the standard entropy of end members and entropies of mixing of solid solutions, are derived. The experimental data can be used further for a number of purposes such as constructing phase diagrams. Figure 1 gives an overview of various mineral end members and solid solutions that have been studied (a list of publications with links to the abstracts of original papers is given below). Quantum-mechanical calculation of heat capacity, using density functional theory (DFT), is another research focus. Calculations are undertaken in order to better understand the microscopic origins of the calorimetrically determined heat capacity behavior.
Methods:
- Low-temperature calorimetry from 2 to 400 K on mg-sized samples using the Physical Properties Measurement System (PPMS).
- High-temperature calorimetry on mg-sized samples using Differential Scanning Calorimetry (DSC) from 278 to 800 K.
- Simultaneous thermal analysis (STA) in cooperation with the working group of Prof. N. Hüsing.
- High-pressure/high-temperature devices including hydrothermal apparatus, piston-cylinder apparatus, and 1 bar furnaces.
- Quantum-mechanical calculations of solid-state properties: MaterialsStudio software implemented at the high-performance computing center (“Doppler Supercomputer”) at the Department of Scientific Computing.
Projects funded by the austrian science fund (FWF) since 2003:
- P15880 (E. Dachs, 2003-2006): Low-temperature heat capacities and entropies of minerals
- P20210 (E. Dachs, 2007-2010): Improvement of two-feldspar thermometry
- P19268 (C. Bertoldi, 2007-2010): Heat capacity measurements at low temperatures 5-300K
- P21370 (E. Dachs, 2010-2013): Calorimetric study of garnet solid solutions
- P23056 (A. Benisek; 2011-2014): Excess-heat capacity of ordered feldspars
- P25597 (C.A. Geiger, 2013-2016): Crystal-chemical and thermodynamic properties of garnet
- P28724 (E. Dachs, 2016-2019): A calorimetric study of biotite solid solutions
- P30977 (C.A. Geiger, 2017-2021): Gesteinsbildende Fe2+-Mg-Silikat-Mischkristalle
Sum of funding: € 2.244.000,–
Publications:
Number of publications since 2005 in international peer-reviewed journals: 69
Petrological software:
PET: Petrological Elementary Tools for Mathematica®
Bachelor and master thesis:
- Baumgartner I. A. (2009) Wärmekapazitäten und Standardentropien wichtiger Amphibolendglieder: Eine kalorimetrische Studie. Master Thesis, University of Salzburg, 94 p.
- Salihović M. (2013) Exzessentropie im System Cu-Zn. Bachelor Thesis, University of Salzburg, 45 p.
- Paunović A. (2013) Untersuchung der Wärmekapazität und Entropie im System Cu-Zn. Bachelor Thesis, University of Salzburg, 45 p.
- Maier M.E. (2013) Exzessentropie von Legierungen in den Systemen Pt-Rh und Ag-Cu. Master Thesis, University of Salzburg, 161 p.
- Stelzhammer M.-C. (2014) Wärmekapazität und Entropie im System Pt-Ir. Bachelor Thesis, University of Salzburg, 48 p.
- Künzel, R. (2016) Entwicklung einer neuen Methode zur Messung der spezifischen Wärmekapazität von Dämmstoffen an ganzen Platten. Bachelor Thesis, University of Salzburg, 68 p.
- Poller J. (2017) Thermodynamische Messungen an Biotit. Bachelor Thesis, University of Salzburg, 45 p.
- Hayböck S. (2018) Abhängigkeit der Sprungtemperatur von supraleitendem Nb3Sn von der thermischen Vorbehandlung. Bachelor Thesis, University of Salzburg, 34 p.





