DigiTherapeutX: Predictable quality of biopharmaceuticals
Team of four research groups at the Paris Lodron University of Salzburg, TU Wien, and BOKU University are involved in elaborating a better understanding and design of bioprocesses. The major aim of the collaboration is the production of biotherapeutics of predictable quality, safety, and efficacy.
Eight of the ten most important pharmaceutical products in 2016 were biopharmaceuticals – drugs obtained from biological sources, mostly by means of culture of mammalian cells.
Process-parameters for a better quality
The industrial production of biopharmaceuticals through cell culture involves the selection of suitable cells as well as the optimization of the conditions for cell culture.
In this context, not only productivity and viability of the host cells are pivotal, but also the molecular structure and purity of the target product (produced in such cells) are critical to ensure therapeutic safety and efficacy of the drug.
The basic idea
The structure of the target product may be described by means of so-called quality attributes, which very strongly depend on cell culture conditions. Therefore, tight process control strategies via suitable, so-called process parameters are obligatory.
Through simultaneous monitoring of quality attributes and process parameters, we plan to elaborate digital models of bioprocesses. Deploying these models, bioprocess parameters may be immediately adapted in the future in order to obtain the desired quality attributes immediately in the course of the bioprocess.
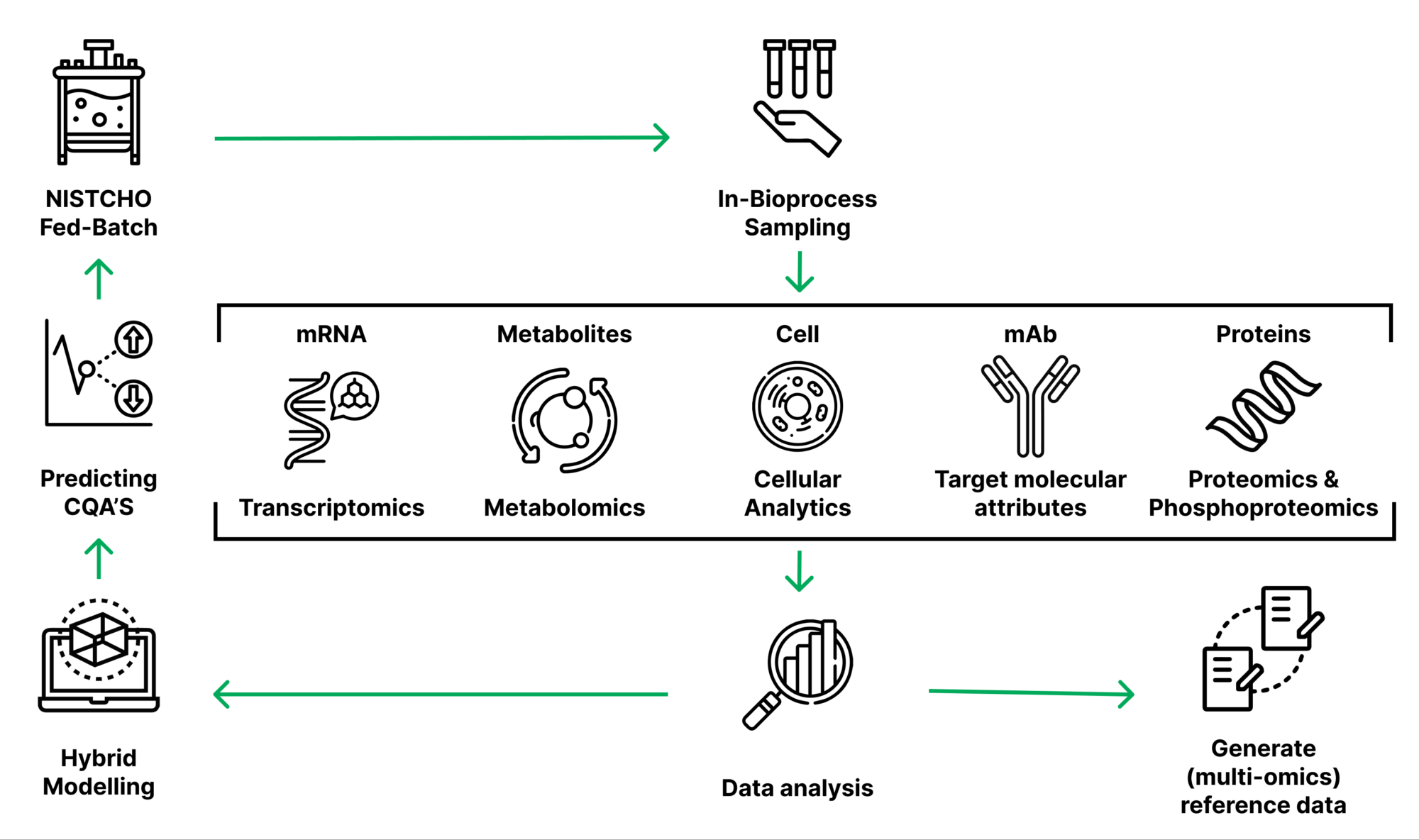
Deployment of mathematical models
Computer-based modeling represents the prerequisite for such immediate bioprocess control.
For the first time, the research team attempts to connect and predict bioprocess parameters and quality attributes systematically via digital, mathematical models. This will be facilitated by the unique acquisition and combination of process data, data about the producing cells, and quality attributes of the target product.
Apart from predictable quality, safety, and efficacy of the product, this will also result in more labor- and cost-effective production of biotherapeutics.
______________________________





