Die AG Cabrele
Forschungsthemen
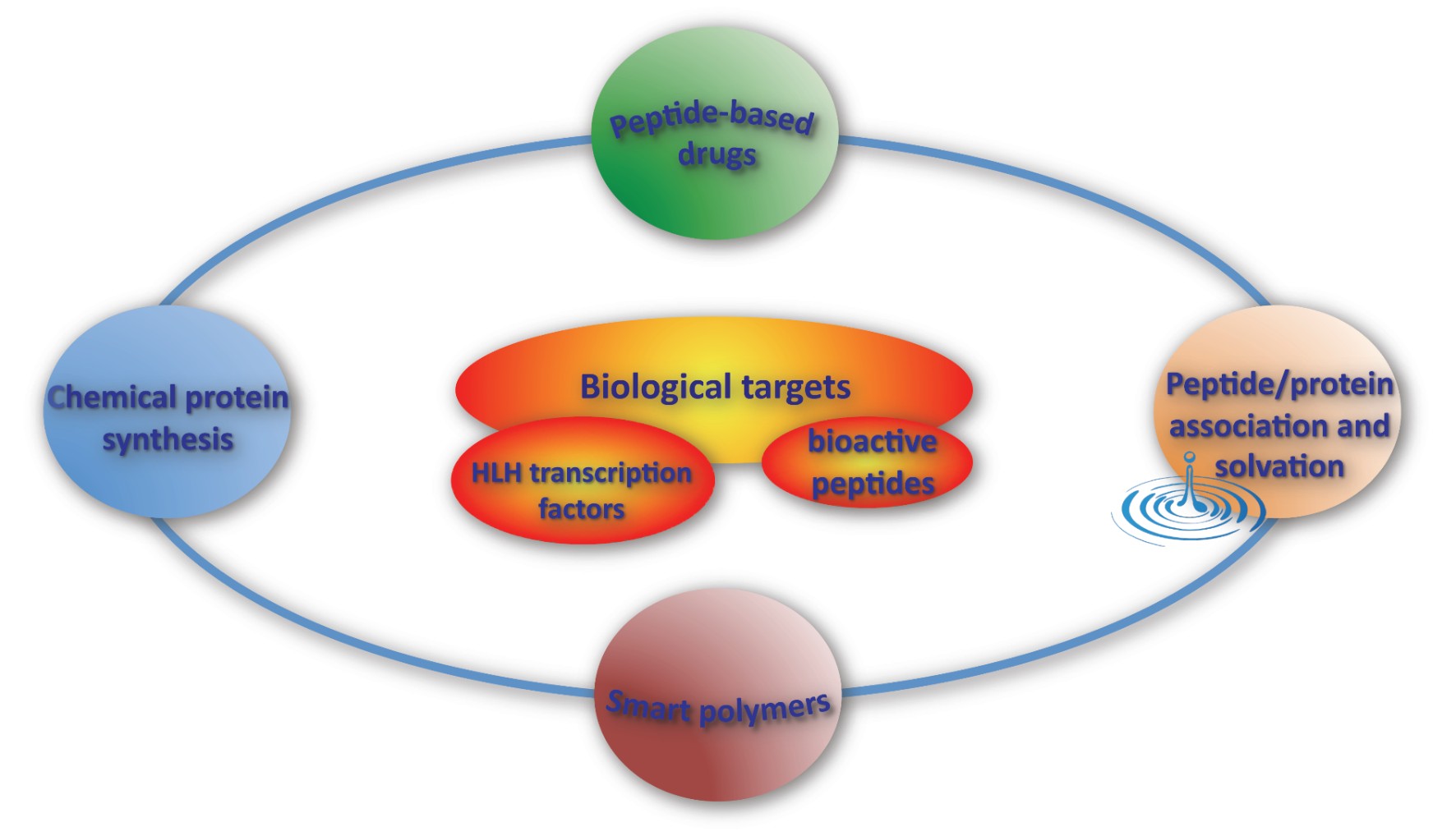
biological targets
Further, we collaborate with colleagues from the University of Regensburg (Germany) to develop analogs of the natural peptide hormones NPY and hPP with selective agonistic/antagonistic binding to their G-protein coupled receptors (the NPY Yn-receptors).
Selected publications:
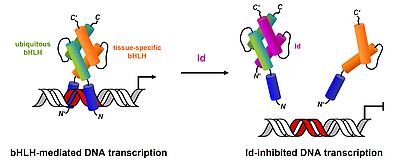
The Recombinant Inhibitor of DNA Binding Id2 Forms Multimeric Structures via the Helix-Loop-Helix Domain and the Nuclear Export Signal, C. Roschger, M. Schubert, C. Regl, A. Andosch, A. Marquez, T. Berger, C.G. Huber, U. Lütz-Meindl, C. Cabrele (2018) Int. J. Mol. Sci. 19(4). pii: E1105. doi: 10.3390/ijms19041105
Synthesis and Conformational Properties of Protein Fragments Based on the Id Family of DNA-Binding and Cell-Differentiation Inhibitors, S. Kiewitz, C. Cabrele (2005) Biopolymers (Peptide Sci.) 80, 762-774.
Synthesis and Conformational Analysis of Id2 Protein Fragments: Impact of Chain Length and Point Mutations on the Structural HLH Motif, N. Colombo, C. Cabrele (2006) J. Peptide Sci. 12, 550-558.
Stepwise Solid-Phase-Synthesis and Spontaneous Homodimerization of the Helix-Loop-Helix Protein Id3, J. Svobodová, C. Cabrele (2006) ChemBioChem 7, 1164-1168.
Synthesis and Conformation of an Analog of the Helix-Loop-Helix Domain of the Id1 Protein Containing the O-Acyl Iso-Prolyl-Seryl Switch Motif, M. Beißwenger, T. Yoshiya, Y. Kiso, C. Cabrele, (2010) J. Peptide Sci. 16, 303-308.
Wenn Proteine ihren Partner wechseln (When proteins chainge their partner), C. Cabrele (2011) BIOSpektrum (Spektrum Akademischer Verlag) 1, 12.
G protein-coupled receptors function as logic gates for nanoparticle binding and cell uptake, W. Hild, K. Pollinger, A. Caporale, C. Cabrele, M. Keller, N. Pluym, A. Buschauer, R. Rachel, J. Tessmar, M. Breunig, A. Goepferich, (2010) Proc. Natl. Acad. Sci. USA 107, 10667-10672.
chemical protein synthesis
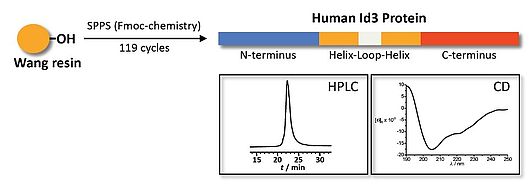
Selected publications:
Synthesis and Conformational Analysis of Id2 Protein Fragments: Impact of Chain Length and Point Mutations on the Structural HLH Motif, N. Colombo, C. Cabrele (2006) J. Peptide Sci. 12, 550-558.
Stepwise Solid-Phase-Synthesis and Spontaneous Homodimerization of the Helix-Loop-Helix Protein Id3, J. Svobodová, C. Cabrele (2006) ChemBioChem 7, 1164-1168.
PEptide-based drugs
Selected publications:
Side-Chain Cyclization Based on Serine Residues: Synthesis, Structure and Activity of a Novel Cyclic Analogue of the Parathyroid Hormone Fragment 1-11, A. Caporale, M. Sturlese, L. Gesiot, F. Zanta, A. Wittelsberger, C. Cabrele, (2010) J. Med. Chem. 53, 8072-8079.
peptide/protein association and solvation
The conformation of a protein depends not only on its primary structure, but also on extrinsic factors such as temperature, ionic strength, pH and hydrophobicity of the solvent. Due to their biological relevance and to their application in different fields spreading from drug formulation to bionanotechnology, it is worthwhile to investigate the behavior of proteins upon environmental changes. Based on the use of peptide models, we are interested in the elucidation of the interplay between different secondary structure motifs and surrounding molecules from the solvent and other additives (collaboration within the Excellence Cluster RESOLV of the Ruhr-University Bochum).

Selected publications:
Recognition of the Helix-Loop-Helix Domain of the ld Proteins by an Artificial Luminescent Metal Complex Receptor, S. D. Kiewitz, M. Kruppa, A. Riechers, B. König, C. Cabrele (2008) J. Mol. Recognit. 21, 79-88.
Synthetic Peptides Containing a Conserved Sequence Motif of the Id Protein Family Modulate Vascular Smooth Muscle Cell Phenotype, S. Pellegrino, N. Ferri, N. Colombo, E. Cremona, A. Corsini, R. Fanelli, M. L. Gelmi, C. Cabrele (2009) Bioorg. Med. Chem. Lett. 19, 6298-6302.
How Ionic Liquids Can Help to Stabilize Native Proteins, H. Weingärtner, C. Cabrele, C. Herrmann (2011) Phys. Chem. Chem. Phys. 14, 415-426.
smart polymers (collaboration with prof. g. ciardelli)
We collaborate with Prof. Gianluca Ciardelli (Politecnico di Torino, Italy) to develop chemical methods for the functionalization of polyurethanes with bioactive amino-acid sequences, aiming at the preparation of biodegradable polymers mimicking the properties of the extracellular matrix. Moreover, we collaborate in the structural characterization of biomimetic polymers for biomedical applications.
Selected publications:
Biomimetic Soluble Collagen Purified from Bones, A. M. Ferreira, P. Gentile, S. Sartori, C. Pagliano, C. Cabrele, V. Chiono, G. Ciardelli (2012) Biotechnol. J. doi: 10.1002/biot.201200184.




