Ongoing projects
completed projects
The projects listed below have been completed in the year 2020 or earlier. Title Carbohydrate coupling of proteins – implications on immune polarization and allergenicity FWF project number W1213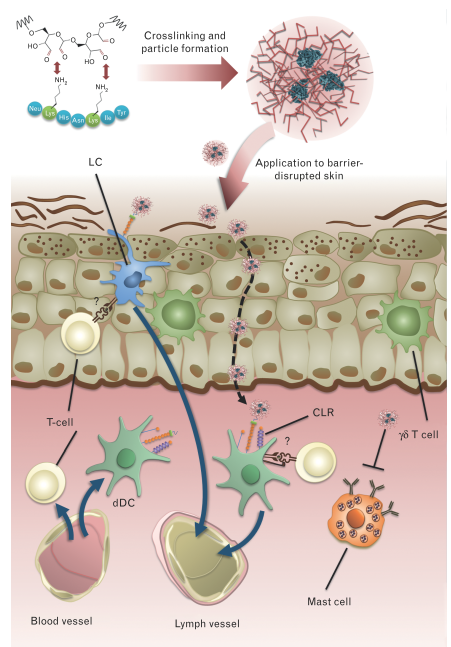 Dendritic cells (DCs) play a central role in initiating and shaping immune responses. They link innate and adaptive immunity as they process and present antigens to B and T cells for their activation. Moreover, the nature of the T helper response is determined by the DCs trough their interaction with T cell during activation. It has been shown that different subsets of DCs can elicit different immune Th patterns, i.e. CD8α+ DCs are prone to drive Th1 and CTL while CD8α- favour Th2 immune responses. Therefore, targeting of antigens to different subsets of DCs represents an attractive approach for vaccination. In this regard, several attempts have been made, from fusion proteins to liposomal preparations with antibodies specific for dendritic cell proteins.
The C-type lectin receptor family (CLRs) encompasses endocytic receptors present on the surface on several immune cell populations including DCs. These CLRs are pattern recognition receptors (PRR) which recognize different carbohydrate structures commonly found in microbes and mediates pathogen endocytosis and production of inflammatory cytokines. We have recently demonstrated efficient targeting of DCs through CLRs using neoglycoconjugates of OVA and Papain with mannan from yeast cell wall. Antigen uptake by DCs was significantly higher for glycoconjugates compared to native protein. Interestingly, in contrast to soluble antigen which induced IgG, as well as robust IgE responses, both OVA and Papain neoglycoconjugates showed even higher IgG titers, however no IgE antibodies were induced. These data indicate a potential effect of neoglycoconjugates on B-cell class switch via CLR ligation. Finally, despite their ability to induce higher IgG titers compared to soluble antigen, papain (but not OVA) neoglycoconjugates showed reduced cross-linking capacity of basophil-bound IgE, making neoglycoconjugates of allergens interesting candidates for specific immunotherapy (SIT) of allergies.
To date, SIT is the only therapeutic intervention for type I allergic patients. However, major limitations of SIT are local or systemic side effects induced by cross-linking of cell-bound IgE, andtherapy associated boosting of systemic Th2 immunity. The latter problem has recently been overcome using transcutaneous immunotherapy via laser microporation instead of conventional subcutaneous route for desensitization. In a mouse model of allergic asthma, immunization via laser-microporated skin revealed a downregulation of Th1/Th2/Th17 responses and an increase of FOXP3+ CD4+ T cells. Epicutaneous immunotherapy has recently been evaluated in a clinical-trial using allergen extract on tape stripped skin. While demonstrating efficacy, local side effects such as itching or eczema have been observed. These results indicate that application of hypoallergenic allergen derivatives may be a pre-requisite for SIT via the skin.
Combining a transcutaneous vaccination approach via laser-porated skin with hypoallergenic neoglycoconjugate vaccines specifically targeting skin DCs, will allow for novel specific immunotherapy protocols with enhanced efficacy as well as safety.
Aims
– Target allergens to specific DC subsets through CLRs
– Evaluate the ability for polarization of the immune response by different
neoglycoconjugates.
– Evaluate hypoallergenic neoglycoconjugates for specific immunotherapy.
Period October 2012 -2020
Title
The influence of fold stability on the immunogenic and allergenic properties of proteins – a focus on immune response polarization
(FWF-project # P26997-B13, project leader Richard Weiss)
Dendritic cells (DCs) play a central role in initiating and shaping immune responses. They link innate and adaptive immunity as they process and present antigens to B and T cells for their activation. Moreover, the nature of the T helper response is determined by the DCs trough their interaction with T cell during activation. It has been shown that different subsets of DCs can elicit different immune Th patterns, i.e. CD8α+ DCs are prone to drive Th1 and CTL while CD8α- favour Th2 immune responses. Therefore, targeting of antigens to different subsets of DCs represents an attractive approach for vaccination. In this regard, several attempts have been made, from fusion proteins to liposomal preparations with antibodies specific for dendritic cell proteins.
The C-type lectin receptor family (CLRs) encompasses endocytic receptors present on the surface on several immune cell populations including DCs. These CLRs are pattern recognition receptors (PRR) which recognize different carbohydrate structures commonly found in microbes and mediates pathogen endocytosis and production of inflammatory cytokines. We have recently demonstrated efficient targeting of DCs through CLRs using neoglycoconjugates of OVA and Papain with mannan from yeast cell wall. Antigen uptake by DCs was significantly higher for glycoconjugates compared to native protein. Interestingly, in contrast to soluble antigen which induced IgG, as well as robust IgE responses, both OVA and Papain neoglycoconjugates showed even higher IgG titers, however no IgE antibodies were induced. These data indicate a potential effect of neoglycoconjugates on B-cell class switch via CLR ligation. Finally, despite their ability to induce higher IgG titers compared to soluble antigen, papain (but not OVA) neoglycoconjugates showed reduced cross-linking capacity of basophil-bound IgE, making neoglycoconjugates of allergens interesting candidates for specific immunotherapy (SIT) of allergies.
To date, SIT is the only therapeutic intervention for type I allergic patients. However, major limitations of SIT are local or systemic side effects induced by cross-linking of cell-bound IgE, andtherapy associated boosting of systemic Th2 immunity. The latter problem has recently been overcome using transcutaneous immunotherapy via laser microporation instead of conventional subcutaneous route for desensitization. In a mouse model of allergic asthma, immunization via laser-microporated skin revealed a downregulation of Th1/Th2/Th17 responses and an increase of FOXP3+ CD4+ T cells. Epicutaneous immunotherapy has recently been evaluated in a clinical-trial using allergen extract on tape stripped skin. While demonstrating efficacy, local side effects such as itching or eczema have been observed. These results indicate that application of hypoallergenic allergen derivatives may be a pre-requisite for SIT via the skin.
Combining a transcutaneous vaccination approach via laser-porated skin with hypoallergenic neoglycoconjugate vaccines specifically targeting skin DCs, will allow for novel specific immunotherapy protocols with enhanced efficacy as well as safety.
Aims
– Target allergens to specific DC subsets through CLRs
– Evaluate the ability for polarization of the immune response by different
neoglycoconjugates.
– Evaluate hypoallergenic neoglycoconjugates for specific immunotherapy.
Period October 2012 -2020
Title
The influence of fold stability on the immunogenic and allergenic properties of proteins – a focus on immune response polarization
(FWF-project # P26997-B13, project leader Richard Weiss)
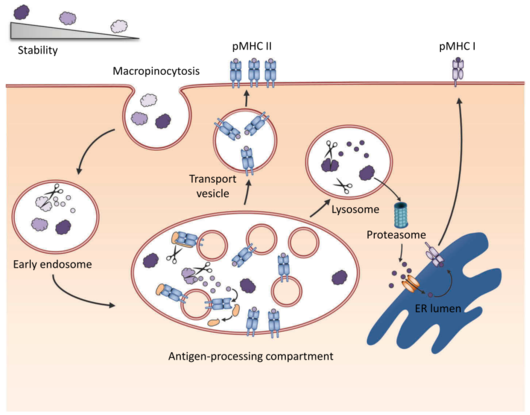 Fold stability influences the immunological properties of proteins via different mechanisms. It impacts lysosomal degradation into T cell epitopes, in vivo survival of antigen and cross-presentation by dendritic cells (DC), and thus can modulate both, induction of antibody responses as well as T cell responses. While recent concepts link protein-intrinsic features, such as protease activity or molecular mimicry, to allergenicity, only few studies focus on the role of protein fold stability on allergenicity, i.e. the potential to induce a T helper 2 (TH2) type immune response including IgE antibody production. Although previous studies have tested the use of various biochemical modifications to modulate structural stability, there is still little known about the immunological effects, and moreover, the results are often contradictory.
This project employs an in silico approach based on knowledge-based potentials to estimate the impact of point mutations on the protein stability. This enables a bi-directional modulation generating both, protein variants with increased as well as decreased stability compared to the wild type molecule. Candidate molecules from this mutation and screening process will undergo a second in silico validation by molecular dynamics simulations. Selected proteins will be expressed, characterized in detail using differential scanning calorimetry (DSC), circular dichroism (CD) spectrum analysis and fourier transform infrared (FTIR) spectroscopy. In silico-generated data will be correlated with empirical structural data using X-ray diffractometry and nuclear magnetic resonance (NMR) spectroscopy. The novel allergen fold stability derivatives will then be analyzed in vitro concerning immunorelevant properties, such as protease resistance, antigen processing, epitope usage and T cell activation and polarization.
In vivo relevance of the concept will be investigated by using adoptive transfer of transgenic T cells specific for non-modulated wild type (WT) molecules, which enables us to study the influence of fold stability during the early events of T cell activation, epitope usage and immune polarization. Immunogenicity and allergenicity of fold-modified proteins will be characterized by subcutaneous and transcutaneous immunization of BALB/c mice. The latter route has been recently identified as a relevant mechanism of natural allergic sensitization. Immune responses will be analyzed by Ig-subclass ELISA, RBL release assay, basophil activation test, proliferation assay, cytokine profiling with Milliplex/Luminex, and morphotyping/intracellular staining.
The results will provide detailed understanding and a comprehensive picture of how protein fold stability acts on critical steps of immune response polarization and allergic sensitization.
Period
November 2014 – 2019
Title
The natural immune response against the timothy grass pollen allergen Phl p 5 in non-atopic humans living in different environments
Associated project in the International PhD Program „Immunity in Cancer and Allergy“ (FWF project number W1213) in collaboration with Rotes Kreuz Oberösterreich
The prevalence of allergic disease is constantly rising in the western world but not in developing countries. The following principal environmental factors have been suggested to underlie this observation: the standard of living, smaller family size, reduced prevalence of infections, the medical treatment, and the hygiene.
The latter has been intensely studied in the so-called “hygiene hypothesis” and has been shown to be a dominant factor for the development of allergic vs. healthy immune responses. It has been postulated that a lack of exposure to infectious agents during early childhood results in a higher risk to develop allergic diseases, whereas a high microbial burden in the first years of life could be crucial for development of a healthy immune response and protection against the development of allergies. The underlying mechanisms presumably rely on viral and bacterial infections, the diversity of microbial components and the genetic background. Farming environment, which is rich in different bacterial compounds, may reduce the risk of atopic sensitization trough an early activation of the innate immune pathways by different pattern recognition receptors (PRR). Activation of the innate immune system has been shown to results in the early establishment of TH1 immunity, secreting IL-12 and IFN-γ, which suppress allergy-promoting TH2 sensitization and IL-4 and IL-13 secretion.
While the allergic immune reaction against pollen allergens has been well characterized in several studies, little is known how the non-atopic immune system deals with allergens. Thus far, at least four immunological outcomes have been described: i) immunological ignorance, ii) induction of regulatory T cells secreting IL-10 (Tr1), iii) immune deviation meaning a shift from a TH2-biased immune response towards a TH1–biased immunity, or iv) induction of blocking antibodies, resulting in the so called modified TH2 response.
APPROACH
In this study, the immune status from non-atopic people, who are regularly exposed to a large number of diverse microbial environments in animal sheds, haylofts and through harvesting activities will be assessed in detail. Furthermore, these results will be compared with the immune status from non-atopic people who are not exposed to these sources of microbial environment.
For this purpose we receive PBMCS from our collaboration partner “Rotes Kreuz Oberösterreich” who screened for non-atopic volunteers living in a farming or urban envorinment. The volunteers had to answer a questionnaire where information regarding their working and living environment was collected. These PBMCs are expanded antigen-specifically with timothy grass pollen allergen Phl p 5 and afterwards T cell function will be determined in terms of surface activation markers, transcription factors, proliferation, mRNA analysis, cytokine secretion and multiple intracellular cytokine secretion.
Period August 2012 -2017
Fold stability influences the immunological properties of proteins via different mechanisms. It impacts lysosomal degradation into T cell epitopes, in vivo survival of antigen and cross-presentation by dendritic cells (DC), and thus can modulate both, induction of antibody responses as well as T cell responses. While recent concepts link protein-intrinsic features, such as protease activity or molecular mimicry, to allergenicity, only few studies focus on the role of protein fold stability on allergenicity, i.e. the potential to induce a T helper 2 (TH2) type immune response including IgE antibody production. Although previous studies have tested the use of various biochemical modifications to modulate structural stability, there is still little known about the immunological effects, and moreover, the results are often contradictory.
This project employs an in silico approach based on knowledge-based potentials to estimate the impact of point mutations on the protein stability. This enables a bi-directional modulation generating both, protein variants with increased as well as decreased stability compared to the wild type molecule. Candidate molecules from this mutation and screening process will undergo a second in silico validation by molecular dynamics simulations. Selected proteins will be expressed, characterized in detail using differential scanning calorimetry (DSC), circular dichroism (CD) spectrum analysis and fourier transform infrared (FTIR) spectroscopy. In silico-generated data will be correlated with empirical structural data using X-ray diffractometry and nuclear magnetic resonance (NMR) spectroscopy. The novel allergen fold stability derivatives will then be analyzed in vitro concerning immunorelevant properties, such as protease resistance, antigen processing, epitope usage and T cell activation and polarization.
In vivo relevance of the concept will be investigated by using adoptive transfer of transgenic T cells specific for non-modulated wild type (WT) molecules, which enables us to study the influence of fold stability during the early events of T cell activation, epitope usage and immune polarization. Immunogenicity and allergenicity of fold-modified proteins will be characterized by subcutaneous and transcutaneous immunization of BALB/c mice. The latter route has been recently identified as a relevant mechanism of natural allergic sensitization. Immune responses will be analyzed by Ig-subclass ELISA, RBL release assay, basophil activation test, proliferation assay, cytokine profiling with Milliplex/Luminex, and morphotyping/intracellular staining.
The results will provide detailed understanding and a comprehensive picture of how protein fold stability acts on critical steps of immune response polarization and allergic sensitization.
Period
November 2014 – 2019
Title
The natural immune response against the timothy grass pollen allergen Phl p 5 in non-atopic humans living in different environments
Associated project in the International PhD Program „Immunity in Cancer and Allergy“ (FWF project number W1213) in collaboration with Rotes Kreuz Oberösterreich
The prevalence of allergic disease is constantly rising in the western world but not in developing countries. The following principal environmental factors have been suggested to underlie this observation: the standard of living, smaller family size, reduced prevalence of infections, the medical treatment, and the hygiene.
The latter has been intensely studied in the so-called “hygiene hypothesis” and has been shown to be a dominant factor for the development of allergic vs. healthy immune responses. It has been postulated that a lack of exposure to infectious agents during early childhood results in a higher risk to develop allergic diseases, whereas a high microbial burden in the first years of life could be crucial for development of a healthy immune response and protection against the development of allergies. The underlying mechanisms presumably rely on viral and bacterial infections, the diversity of microbial components and the genetic background. Farming environment, which is rich in different bacterial compounds, may reduce the risk of atopic sensitization trough an early activation of the innate immune pathways by different pattern recognition receptors (PRR). Activation of the innate immune system has been shown to results in the early establishment of TH1 immunity, secreting IL-12 and IFN-γ, which suppress allergy-promoting TH2 sensitization and IL-4 and IL-13 secretion.
While the allergic immune reaction against pollen allergens has been well characterized in several studies, little is known how the non-atopic immune system deals with allergens. Thus far, at least four immunological outcomes have been described: i) immunological ignorance, ii) induction of regulatory T cells secreting IL-10 (Tr1), iii) immune deviation meaning a shift from a TH2-biased immune response towards a TH1–biased immunity, or iv) induction of blocking antibodies, resulting in the so called modified TH2 response.
APPROACH
In this study, the immune status from non-atopic people, who are regularly exposed to a large number of diverse microbial environments in animal sheds, haylofts and through harvesting activities will be assessed in detail. Furthermore, these results will be compared with the immune status from non-atopic people who are not exposed to these sources of microbial environment.
For this purpose we receive PBMCS from our collaboration partner “Rotes Kreuz Oberösterreich” who screened for non-atopic volunteers living in a farming or urban envorinment. The volunteers had to answer a questionnaire where information regarding their working and living environment was collected. These PBMCs are expanded antigen-specifically with timothy grass pollen allergen Phl p 5 and afterwards T cell function will be determined in terms of surface activation markers, transcription factors, proliferation, mRNA analysis, cytokine secretion and multiple intracellular cytokine secretion.
Period August 2012 -2017
Title P.L.E.A.S.E. Vaccinate: Transcutaneous Immunization of Licensed Vaccines via Laser-generated Micropores – a Comparative Study (industrial project, funded by Pantec Biosolutions) Today, the majority of vaccines is administered by the intramuscular route using hypodermic needles and syringes, even though muscle is not a highly immunogenic organ. Development of effective methods for vaccine delivery to the skin is considered a feasible approach. The skin represents an important peripheral immune organ attractive for vaccination as it is rich in immunocompetent cells including Langerhans cells, dermal dendritic cells and keratinocytes, and its efficient drainage to lymph nodes. Recent studies have demonstrated that vaccination via the skin results in better antigen trafficking into lymph nodes compared to intramuscular injection. Following skin immunization, Langerhans cells and dermal dendritic cells residing in the epidermal and the dermal compartment of the skin, respectively, play an important role in antigen processing and presentation. Additionally, other skin resident cells, including keratinocytes, potentiate and control immune responses by cytokine and chemokine production. Many clinical studies demonstrated the efficacy of intradermal delivery, some of them showing dose-sparing effects or improved protective immunity in the elderly. However, administration of antigens to the dermal layer of the skin using hypodermic needles is painful, needs specially trained medical personnel, and typically achieves inconsistent delivery. Additionally, local reactions at the injection site are more frequently found after intradermal delivery. An ideal skin vaccination method should therefore be reliable, but also eliminate the dangers and pain associated with hypodermic needles. Introduction of a methodology to circumvent the Stratum corneum in a highly reproducible, but also adaptable manner would be desirable. Given the precision of the laser scanning technique together with the flexibility in depth, number and density of the produced micropores provided by the P.L.E.A.S.E. vaccinate platform, this device completely fulfills these criteria. Using carefully selected vaccine candidates, we will provide proof of concept pre-clinical data for P.L.E.A.S.E. vaccinate as a pre-requisite for further clinical evaluation. Period May 2012 -2016
Title Evaluation of the usability of a therapeutic mouse model for B-cell peptide based immunotherapy vaccines (industrial project, funded by Biomay) Description of the project BM32 is a mixture of four recombinant proteins, engineered to present linear B-cell epitopes in the context of the Hepatitis B virus pre-S protein. Epitopes are derived from the timothy grass pollen allergens Phl p 1, Phl p 2, Phl p 5, and Phl p 6 and the corresponding recombinant proteins are termed BM321, BM322, BM325, and BM326 respectively. The alum adsorbed mixture of the 4 proteins has been demonstrated to elicit potent antibody responses that displayed IgE blocking capacity in vitro. BM32 is supposed to elicit high levels of allergen specific IgG antibodies that prevent cross-linking of mast cell bound IgE upon allergen encounter, and IgE mediated allergen uptake by dendritic cells. In this project, BM32 will be evaluated in a therapeutic setting utilizing an established Balb/c mouse model of allergic asthma. This mouse model has been previously used to evaluate subcutaneous immunotherapy (SCIT) using the recombinant allergen Phl p 5. rPhl p 5 SCIT significantly reduced airway hyperresponsiveness, cellular infiltration of the lung, and local production of cytokines in the lung. Protection was associated with increased secretion of IL-10 by allergen re-stimulated splenocytes, suggesting immune modulation on the T cell level. The current study will demonstrate whether induction of blocking antibodies alone is sufficient to reduce allergic symptoms and induce systemic immune deviation. Period May 2012 – June 2014
Title Laser-poration for transdermal allergen immunotherapy (FWF project number P21125-B13, Project leader Sandra Scheiblhofer ) Description of the project For almost a century, allergen immunotherapy has been used as the only antigen-specific immunomodulatory therapy for allergic diseases. Allergen extracts or recombinant allergens are administered either by subcutaneous injection (SCIT) or, more recently, via the sublingual route (SLIT). SCIT requires weekly injections over months or even years and can potentially cause severe side effects. In contrast, SLIT appears to be a safer, but less efficient alternative that requires higher doses and even more frequent (daily) applications because antigen uptake via the mucosa is limited. Divided into two areas – the epidermis and the dermis – the skin is considered a promising target for immunotherapy because it is rich in antigen presenting cells. Both layers are populated by different subsets of skin derived dendritic cells, which differ in surface markers and their immunological properties. Langerhans cells in the epidermis are commonly believed to represent the first line of defence against pathogens. However, recent studies suggest a more regulatory role for Langerhans cells and that instead dermal dendritic cells residing in the dermis are crucial for the development of cutaneous immune reactions. In the proposed project, we intend to precisely target allergen-encoding plasmid DNA or recombinant allergens either to the epidermis or to the dermis of mice. This will be achieved by using a proprietary laser device developed by Pantec Biosolutions, which enables skin ablation in 5-10 micrometer steps, thereby creating controlled aqueous micropores allowing for high diffusion rates over prolonged periods of time (48h). Vaccine formulations applied to such pore arrays are taken up at similar levels compared to subcutaneous injections. For increased patient compliance and long term exposure of the pore arrays with the respective DNA-based or recombinant vaccine, dermal patches incorporating the vaccine in a gel formulation will be developed. This technology enables more efficient immunostimulation, and therefore reduces the necessary application frequency compared to SLIT, while retaining high patient compliance associated with a painless method that can be applied by the patient at home. Detailed characterization of the immune responses elicited with the various vaccines targeting the two different skin compartments will be performed. The relevance of Langerhans cells for the induction of immune responses will be investigated using transgenic mice. Finally, after establishing the optimal parameters for laser settings and vaccine formulations, we will investigate the applicability of this novel vaccine delivery platform for specific immunotherapy in our well established mouse model of type I allergy. Period January 2009-December 2012
Title Mapping of helper T-cell epitopes of the grass pollen allergen Phl p 5 (industrial project, funded by Biomay) Description of the project Allergen gene vaccines as well as allergen protein vaccines (e.g. as used in specific immunotherapy-SIT) display their anti-allergic activity via specific induction of T-cells. In the case of gene vaccines, both, the prophylactic and therapeutic efficacy is based on CD+ T-cells of the Th1 type. On the other hand, successful immunotherapy with protein vaccines is assumed to be based on a combination of regulatory T-cells (inducing a tolerant state of the immune system), IFN-gamma-producing T-cells (deviating the Th2 type immune response) and IgG antibodies binding to the allergen and thus blocking the crosslinking of IgE on mast cells and basophils. A necessary prerequisite for an anti-allergic vaccine would be that all three mentioned mechanisms work in an allergen-specific way, which requires the induction of allergen-specific T-cells. Therefore, the knowledge of T-cell epitopes is crucial for the development and testing of gene and protein vaccines for the treatment of type I allergy. The project will strive for the characterization of mouse and human helper T-cell epitopes of the grass pollen allergen Phl p 5. Period November 2007 – December 2009
Title Systematic evaluation of up-to-date genetic vaccination approaches for protective and therapeutic treatment of type I allergy: Preparation of optimized vaccines for phase I clinical trials (FWF project number L181 B13) Description of the Project The prevalence of allergic diseases has increased substantially over the past few decades in the industrialized world despite the introduction of new and effective drugs for their treatment. At present, allergic diseases affect more than one quarter of the population. Allergy can be defined as a disorder of the immune system, leading to inappropriate responses against commonly encountered substances that are otherwise harmless. Clinically, the most common allergic diseases are asthma, rhinitis/conjunctivitis (hayfever) and eczema. Allergic immune responses are characterized by the synthesis of allergen-specific IgE antibodies and the production of immunomodulatory molecules such as interleukin (IL)-4, IL-5 and IL-13. Today, most anti-allergic treatments only control the symptoms of allergy via immunosuppressive and anti-inflammatory agents such as antihistamines, corticosteroids and beta-agonists. The only curative approach targeting the disease is allergen-specific immunotherapy (SIT). Although SIT has already been introduced by Noon and Freeman in 1911, the immunological mechanisms underlying this treatment are still unclear. Although SIT has proven its suitability and clinical efficacy especially for mono-or oligosensitized patients and for treatment of hypersensitivities against stinging insects, only 30-50% of allergic rhinitis patients respond and SIT is even less effective for asthmatics. Another major disadvantage of classical SIT is the risk of anaphylactic side effects caused by systemic application of large amounts of allergen via subcutaneous injections. In summary, there is an urgent need for improvement and/or alternatives to conventional SIT. Over the last decade, the genetic vaccine revolution has provided researchers with exciting possibilities to design new advanced vaccines. Genetic vaccination employs the allergen in its purest form – the genetic information. Following transfection with the genetic material, cells of the vaccinated individual will translate the information into the respective allergen. This type of vaccine specifically induces a type of immune response dominated by the immunomodulator interferon gamma, which suppresses IL-4/IL-5 dependent allergic immune responses. Meanwhile, a panel of genetic vaccines has been successfully administered in animal models, including plasmid DNA, purified RNA, and „self-replicating“ DNA/RNA. Also several different delivery methods such as intramuscular or intradermal injections, application via „gene gun“, in vivo electroporation, or intranodal and mucosal routes have been investigated in animal models. However, results from early clinical studies (for infectious diseases and cancer) have often been disappointing due to suboptimal utilization of the latest feasibilities of modern genetic vaccines. Although animal studies with anti-allergic genetic vaccines have been promising, a systematic evaluation of different vaccines, doses, immunization methods and schedules will be of crucial importance for the success of future clinial trials. For the present study, we will establish novel anti-allergic RNA vaccines and bacterial ghost-delivered genetic and recombinant vaccines and evaluate them together with the present state-of-the-art genetic vaccines, application methods, adjuvants and immunization protocols as a basis for preparing clinical application. Simultaneously with providing the urgently necessary comparative information about different vaccination approaches, an objective evaluation will also exert gentle pressure on planning of phase I trials, thus helping to speed up the process of developing rational anti-allergic genetic vaccines for human use. Period 1.1.2006 – 31.12.2008
Title Immune responses to antigen application on bare skin (In collaboration with the Bernhard-Nocht-Institute for Tropical Medicine, Hamburg, Germany) Description of the Project The skin represents an important target for gene gun vaccination strategies. However the exact mechanisms that underline the induction of an adaptive immune response induced by that technique are poorly understood. In the present project we analyze different cutaneous dendritic cell subsets accounting for efficient vaccination. This basic research will help us to improve current vaccination strategies. Furthermore, novel concepts are tested whether epicutaneous incorporation of different antigens is able to modulate ongoing immune responses. Period Since 1.1.2005
Title Bacterial ghosts as delivery system for genetic vaccines (In collaboration with the Institute for Microbiology and Genetics of the University of Vienna) Bacterial ghosts are empty gram negative bacterial cellular envelopes with retained morphological, structural and antigenic features of the cell wall. Bacterial ghosts can be used as vaccine candidate per se. Alternatively, they can be loaded with DNA vaccines and recombinant protein vaccines and have been demonstrated to effectively induce cellular and humoral responses, thus introducing a promising technology for the development of novel vaccine types. The mode of application of DNA vaccines is known to be decisive for the immunogenicity of the construct and the induced type of immune response and bacterial danger signals transmitted via pattern recognition receptors (e.g., TLRs) represent strong triggers of immune reactions. In vitro studies with Mannheimia haemolytica ghosts carrying plasmid DNA revealed efficient uptake by APCs, leading to transfection rates up to 60 %. In addition to targeting the DNA vaccine construct to APCs, bacterial ghosts promote maturation and activation of dendritic cells as the encoded antigen is delivered in the context of an adequate danger signal. However, the endotoxic effects of free LPS are not observed, because LPS is associated with the ghost envelopes. In the present project the application of DNA vaccines with, or recombinant antigens fused to bacterial ghosts is evaluated in comparison with conventional protein immunization procedures and the state-of-the-art gene vaccination methods. Period Since 1.1.2005
Title Design-allergens for a DNA-based allergy-vaccine concept (FWF project number S8811) Description of the Project A special feature of genetic immunization with naked DNA vaccines, and the major difference between conventional immunization with protein antigens (or allergens) and plasmid DNA, is the induction of an INF-gamma-mediated Th1 type response with intradermal or intramuscular DNA injection methods. The project intends to take advantage of the characteristics and mechanisms underlying DNA-based immunization, which offer unique approaches for the design and variation of novel vaccines. The principle goal is the development of a panel of newly designed DNA fusion and multi vaccines optimized concerning immunogenicity and Th1 promoting activity against whole groups of allergens. Period 01.01.2001 – 31.12.2005
Title Genetic immunization for the treatment of malaria (In collaboration with the Walter Reed Army Institute of Research, Silver Spring, MD, USA, and the Bernhard-Nocht-Institute for Tropical Medicine, Hamburg, Germany) Description of the Project Today, malaria is a public health problem in more than 90 countries inhabited by 40% of the world’s population. The WHO estimates mortality due to malaria to be over 1.1 million each year with the vast majority of victims among young children in Africa. The causative agents in humans are four species of Plasmodium protozoa – P. falciparum, P. vivax, P. ovale, and P. malariae, transmitted by Anopheline mosquitoes. P. falciparum accounts for most of the infections and is the most lethal. Widespread resistance of P. falciparum to conventional anti-malarial drugs as well as insecticide resistance of mosquitoes has increased the urgency for malaria vaccination. Since the first publications reporting the induction of immune responses by “naked” plasmid DNA, vaccine research has been revolutionized. In a large number of animal studies DNA vaccines have demonstrated their potential to induce both humoral and cellular immune responses, including protection against a variety of viruses, bacteria and parasites, and are currently undergoing phase I clinical trials in humans. Because of its ability to induce the cellular branch of the immune system, DNA immunization also opened new perspectives for the development of a malaria vaccine. Together with our collaboration partners we first focused on the development of protective vaccination approaches against the circumsporozoite protein (CSP) and investigated the protective efficacy of intradermal needle or epidermal gene gun application of plasmid DNA encoding CSP. In this study, we compared for the first time the immunogenicity and efficacy of a malaria DNA vaccine delivered to the same site by different techniques. Although needle injection provides approximately 50 times more plasmid than gene gun immunization, the ballistic delivery of the CSP-encoding vaccine was superior. Gene gun immunization induced higher antibody titers and significantly enhanced protective immunity against a single infected mosquito bite. These findings underscored the importance of the technique used for delivering DNA vaccines versus the choice of the target tissue. In the recent and ongoing work we concentrate at the leading erythrocyte stage antigens, the merozoite surface protein 1 (MSP1) and the apical membrane antigen 1 (AMA1) from P. falciparum, which will be administered via different delivery systems. Additionally, we intend to improve the humoral immunogenicity of MSP1 and AMA1 by fusing them with multiple copies of C3d, which has been demonstrated to be a highly efficient molecular adjuvant. Period since 01.01.1997
Title Development of safety-optimized genetic desensitization protocols with DNA replicons encoding allergens (FWF project number S8813 – in collaboration with the National Institutes of Health, National Cancer Institute, Bethesda, MD, USA) Description of the Project The Th1-biased immune response induced by intradermal or intramuscular injection of plasmid DNA obviously enables to prevent from an allergic reaction as well as to balance an established Th2 type response. However, the major restriction of genetic immunization (especially concerning the clinical use) is the requirement of large doses of DNA. To overcome this problem, the first aspect of the present project will be the application of a novel type of genetic vaccines, the so-called self-replicating DNA vaccines or DNA replicons, for allergy protection and treatment. Intradermal immunization with these constructs induces the desired Th1-biased immune response with only nanogram quantities of injected DNA. The second aspect of the project will cover the development of strategies for minimizing the risk of anaphylactic side effects with forced ubiquitination. Summing up, the project approaches should lead to the development of safety-optimized and effective genetic vaccines for the treatment of type I allergy. Period 01.01.2003 – 31.12.2005
Title Development of malaria DNA vaccines (FWF project number T133 – in collaboration with the Walter Reed Army Institute of Research, Silver Spring, MD, USA, Project leader Sandra Scheiblhofer ) Description of the Project Malaria is by far the world´s most serious tropical parasitic disease, which kills more people than any other communicable disease besides tuberculosis. So far, conventional immunization strategies have yielded disappointing results, but the novel and revolutionary method of DNA-based vaccination has already proven its effectiveness against malaria infection in the mouse model.The aim of the proposed study is to improve our recently developed anti-malaria DNA vaccines, thereby reducing the amount of DNA required for protective immunity as well as the number of booster immunizations, a prerequisite for their application in developing countries. Furthermore, the project investigates the question whether DNA vaccines confer protection via cytotoxic T cell responses and/or antibodies against malaria infection. Period 01.10.2002 – 30.09.2005
Title DNA vaccination against Borreliosis (In cooperation with the Biomedical Research Center, Baxter Vaccines AG, Vienna, Austria) Description of the Project Borreliosis (Lyme disease), the most common vector-borne disease in North America and Europe, is a progressive multisystem illness with clinical manifestations involving skin, joints, heart and nervous system. Borrelia burgdorferi, the spirochetal agent of Lyme disease, is transmitted when infected Ixodes ticks feed on susceptible hosts. The outer surface protein C (OspC) dominates the early stages of infection and Lyme disease patients as well as naturally or experimentally infected mice produce antibodies to OspC. Considering these phenomenons, an OspC-based vaccine should have strong protective effects against spirochetes in the host. DNA-based immunization turned out to be a new and potent method to successfully immunize not only against viruses such as influenza, HIV and Herpes simplex and intracellular pathogens like mycobacteria, but also against parasitic infections including Plasmodium and Leishmaniasis as well as mycoplasmas. In a recent publication we demonstrated a DNA vaccine approach against Borreliosis using a construct encoding the OspC gene. The results indicated that for DNA-based immunization against OspC an ER-targeting signal was necessary for both antibody production as well as cellular immune responses. The present project takes advantage of the fact that different DNA-immunization procedures can result in different types of immune responses. Gene gun administration has been proven to elicit a clear Th-2 type response whereas needle injection induces a strong Th-1 type response. Therefore we intend to modulate the immune response against OspC by the application of these two methods. In addition, the effects of oligodeoxynucleotides containing CpG-motifs, known as potent stimulators of Th-1 type responses are investigated. The combination of both approaches should result in different and clearly distinguishable types of immune responses, which may be the basis for testing and optimizing the effectiveness of a DNA-vaccine against Borreliosis in future protection studies. Period 01.01.1997 – 31.12.2003
Title The influence of genetic immunization on endocrine functions (In cooperation with the Slovak Academy of Sciences, Institute of Experimental Endocrinology, Bratislava, Slovak Republik) Description of the Project The project is dealing with two molecules, the retinoic acid receptor (RAR) and the type I iodothyronine 5´-deiodinase (5‘-DI), which primarily play an important role in hormon regulation but are also involved in immunological reactions. Nuclear retinoid receptors – retinoic acid inducible transcription factors – participate in pathways influencing many components of the immune system. In the present project we investigate in vivo effects of DNA-based immunization of mice on binding parameters of all-trans RARs in spleen cell nuclei. An eucaryotic expression vector encoding the gene for the model enzyme ß-galactosidase of Escherichia coli (pCMV-ß) is used for intradermal injection. Furthermore, immunostimulatory CpG motifs, which may serve as a “danger signal” for the mammalian immune system, are coinjected as oligodeoxynucleotides. The results demonstrate that the concentration of RARs is significantly reduced in the late phase of the primary immune response (21 days after injection of plasmid DNA – indicated by high affinity IgG antibodies and IFN-gamma expression). Coinjection of CpG motifs does not change the course of the humoral response but enhances and accelerates the proliferativ! e response and expression of IFN-g, which correlates with the reduced RARs concentration. The second part of the project deals with the aspect that inflammatory cytokines in vitro are believed to be involved in the regulation of 5‘-DI activity. This study is undertaken to investigate in vivo effects of DNA immunization on the 5´-DI activity in the liver. Again the mammalian expression vector pCMV-ß is used for intradermal immunization and immunostimulatory CpG motifs, which induce the expression of IL-6, IL-12, IL-18, TNF-a/ß and IFN-g are coinjected as oligodeoxynucleotides. From the first data we can conclude that the activity of 5´-DI in mouse liver when compared to non-immunized animals (100 %) is found to be significantly enhanced by DNA immunization two weeks (175.7 %) or three weeks (192.6 %) after the plasmid injection. In addition, the activity of the 5´-DI in mouse liver is markedly enhanced two weeks (252.4 %) or three weeks (243.3 %) after the injection when CpG motifs are applied together with the plasmid DNA. Period 01.01.1997 – 31.12.2003




