Functional Studies on extra-lysosomal Legumain
FWF START Project #Y01469 | Project Leader: Elfriede Dall
Link to project page: Lysosomal Proteases
What is it about?
Normally, the enzyme legumain is found in the endo-lysosome of our cells. There it is an important plyer of our immune system. It cleaves foreign antigens such as toxins into smaller peptides so that they can be recognized by our immune system. Mainly under pathophysiological conditions, legumain has been found mislocalized in the cytosol, nucleus or extracellularly. Although the mislocalization of legumain has been associated with serious diseases such as cancer and Alzheimer’s disease, its function in these atypical locations is still not understood. Because of its relevance in a number of very different diseases, legumain has become an attractive target for drug development. However, to develop safe and efficient drugs, we need a detailed understanding of our target. Therefore, in this project, we aim to investigate the molecular function of mislocalized cytosolic legumain.
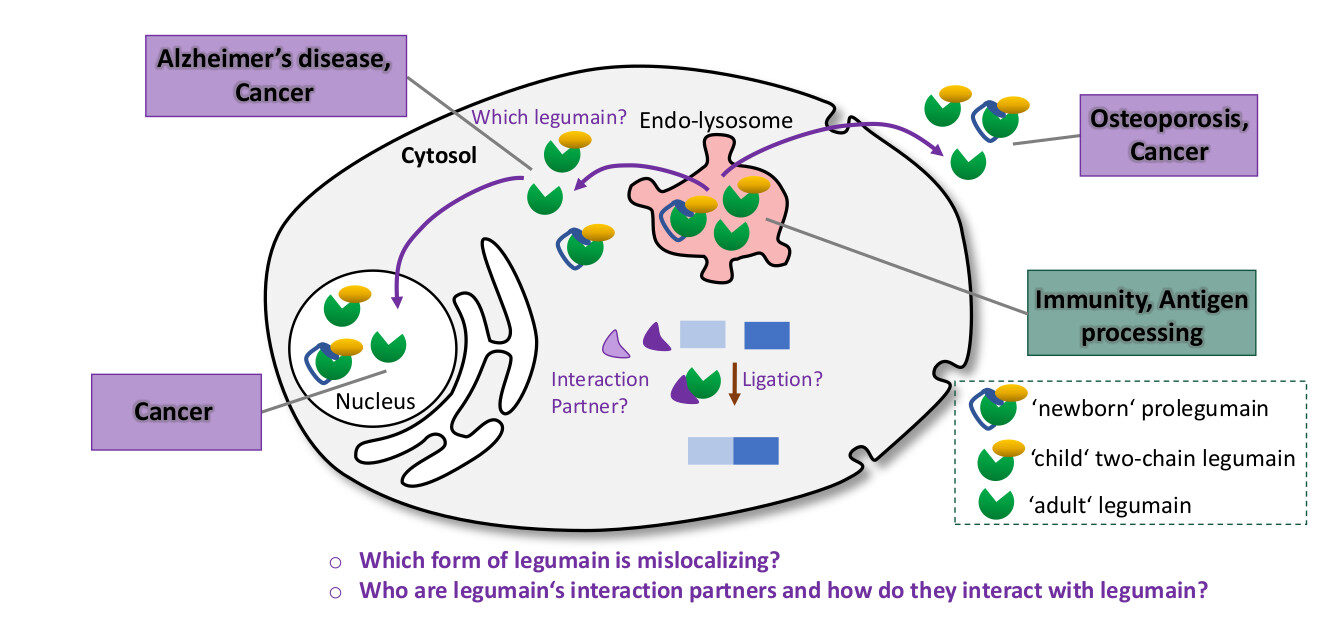
Fig. 1. Extra-lysosomal legumain is associated with different diseases.
In a previous study, we found that legumain exists in three distinct forms corresponding to a newborn, child, and adult form. These different forms of legumain have different functions and different properties. While the adult form survives only in the acidic endo-lysosome, newborn and infantile legumain also tolerate a neutral pH, as found in the cytosol. Furthermore, in addition to their protease function, the newborn and adult forms of legumain also exhibit a ligase function. They can not only cut other proteins, but also link them. Importantly, legumain has the ligase function only at neutral pH.
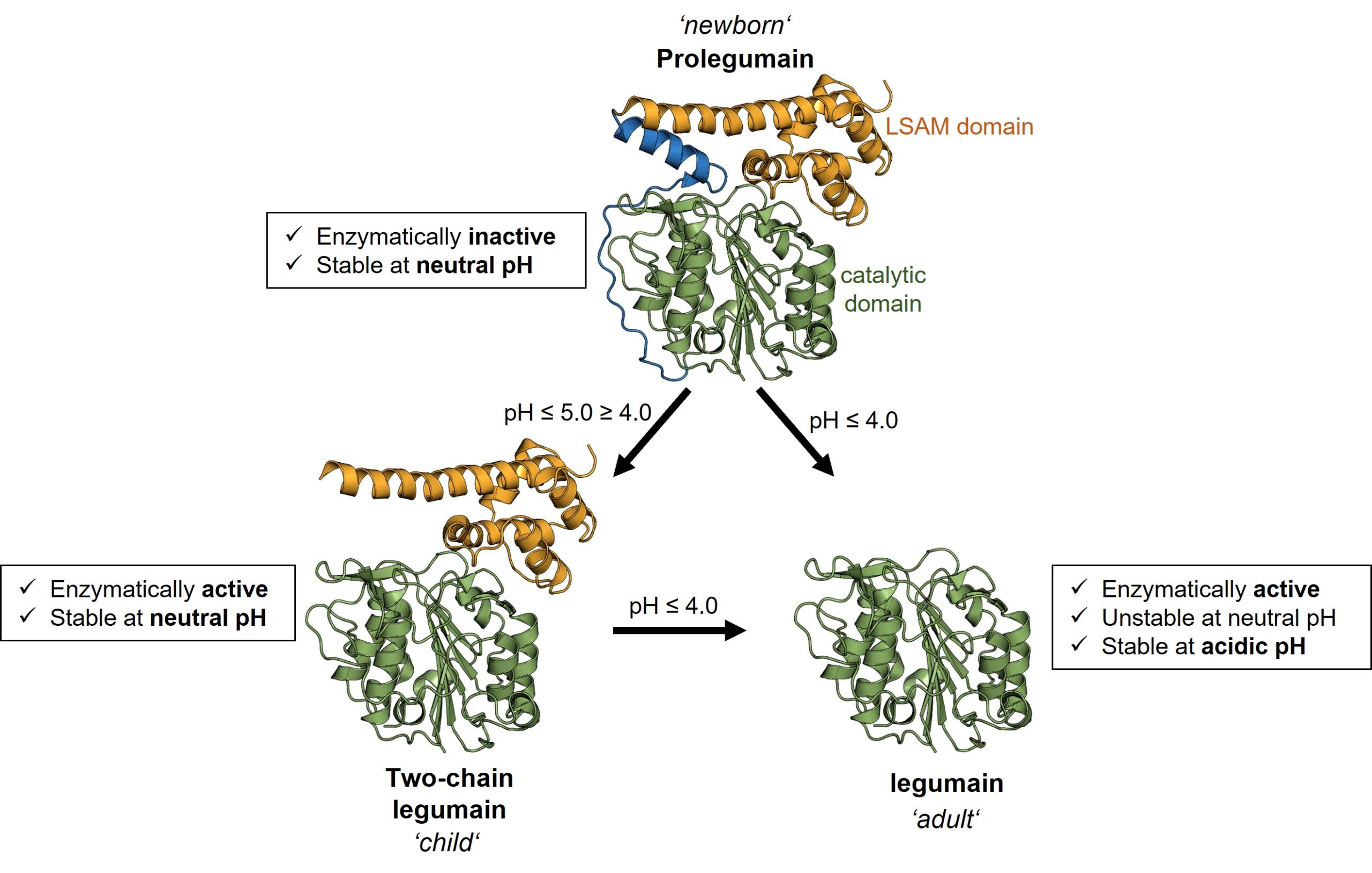
Fig. 2. Legumain exists in 3 different forms with different functions and pH stabilities.
Based on these findings, we hypothesize that:
- different forms of legumain have environment-specific functions,
- when the adult form of legumain is mislocalized, it must be stabilized by an interaction partner, and
- mislocalized legumain exerts its pathophysiological function not only by cutting but also by ligating other proteins.
To test these hypotheses, we aim to develop a method to monitor the ligase function of legumain in real time. We also want to identify which form of legumain is mislocalized under pathophysiological conditions and who its interaction partners are. This knowledge will allow us to unravel the interaction network of mislocalized legumain and subsequently develop new therapeutic and diagnostic tools.





